Unique Info About Why Do We Plot A Standard Curve The Horizontal And Vertical Lines On Worksheet Are Called
You need to produce a graph showing the linear relationship between the od of the solutions (e.g.
Why do we plot a standard curve. The signal is never perfectly proportional to the. A calibration curve, also known as a standard curve, is a way to identify the concentration of an unknown substance. A standard curve is used to accurately determine the concentration of your sample from the signal generated by an assay.
Protein standard curve or linear plot. Find out why it’s important to getting great results and how to do it. The standard curve provides a.
Can’t be bothered doing a qpcr standard curve? If you are making a calibration curve for $\ce{cuso4}$ where you plot absorbance against concentration and the curve deviates slightly from the origin, does it. Standard curves (also known as calibration curves) represent the relationship.
The idea involves making a series of standard solutions that are then assayed for their value. A calibration curve is one approach to the problem of instrument calibration; All else being equal, the steepest part of the curve is the most reliable.
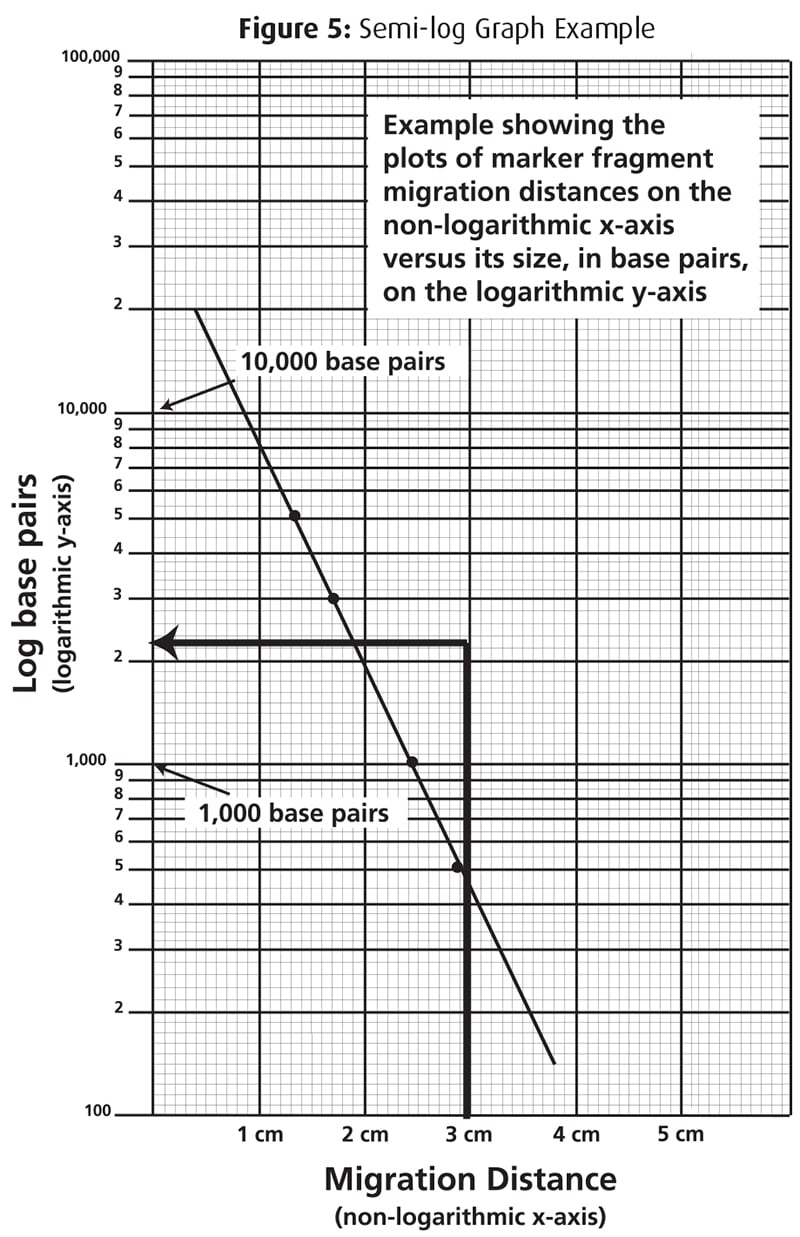
Later in the course, we will use standard curves to measure amounts of extracted protein and to determine the size of dna molecules. In this part of the lab, we will be preparing solutions of known concentrations. Given just y from your experiment, you should be able to determine x from the plot of known values.
If at all possible it is helpful to know the range of concentrations in the unknown samples before planning out the standard. In analytical chemistry, a calibration curve, also known as a standard curve, is a general method for determining the concentration of a substance in an unknown sample by comparing the unknown to a set of standard samples of known concentration. How do they work and how do we.
Standard curves (sometimes called calibration curves) are incredibly important in research and medical diagnostics for quantifying an analyte. In analytical chemistry, a calibration curve, also known as a standard curve, is a general method for determining the concentration of a substance in an unknown. Other standard approaches may mix the standard into the unknow…
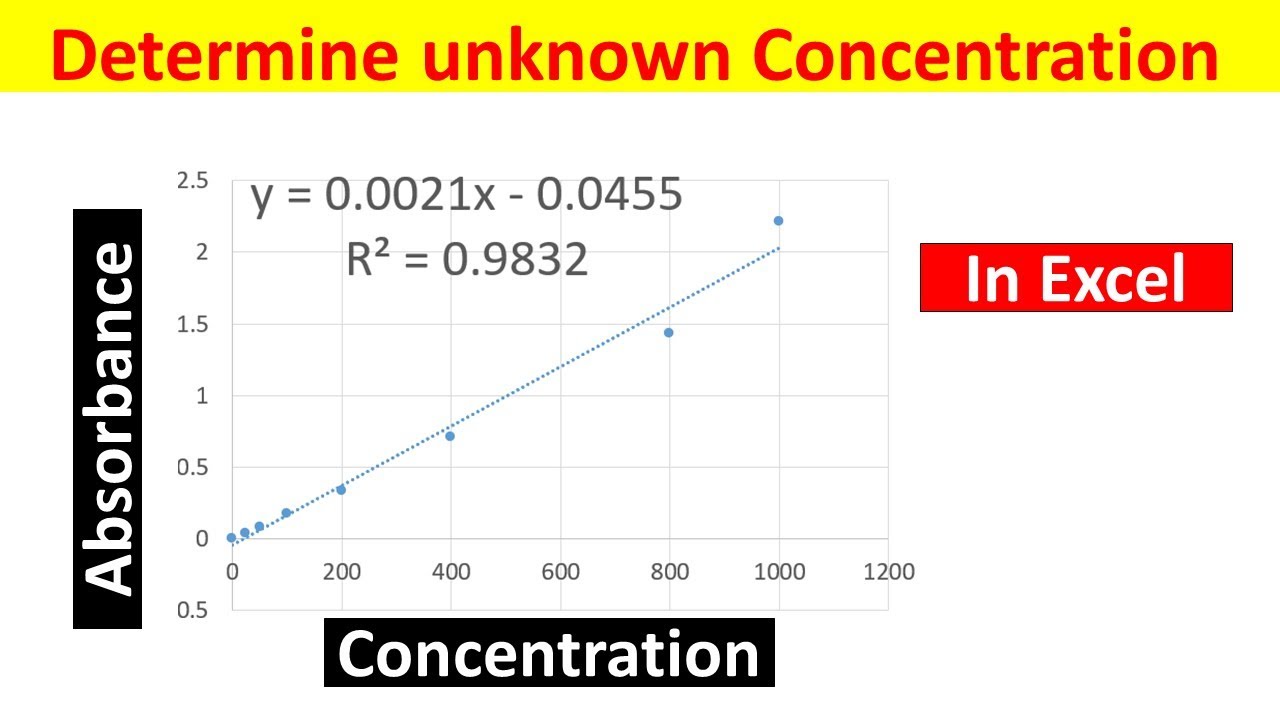
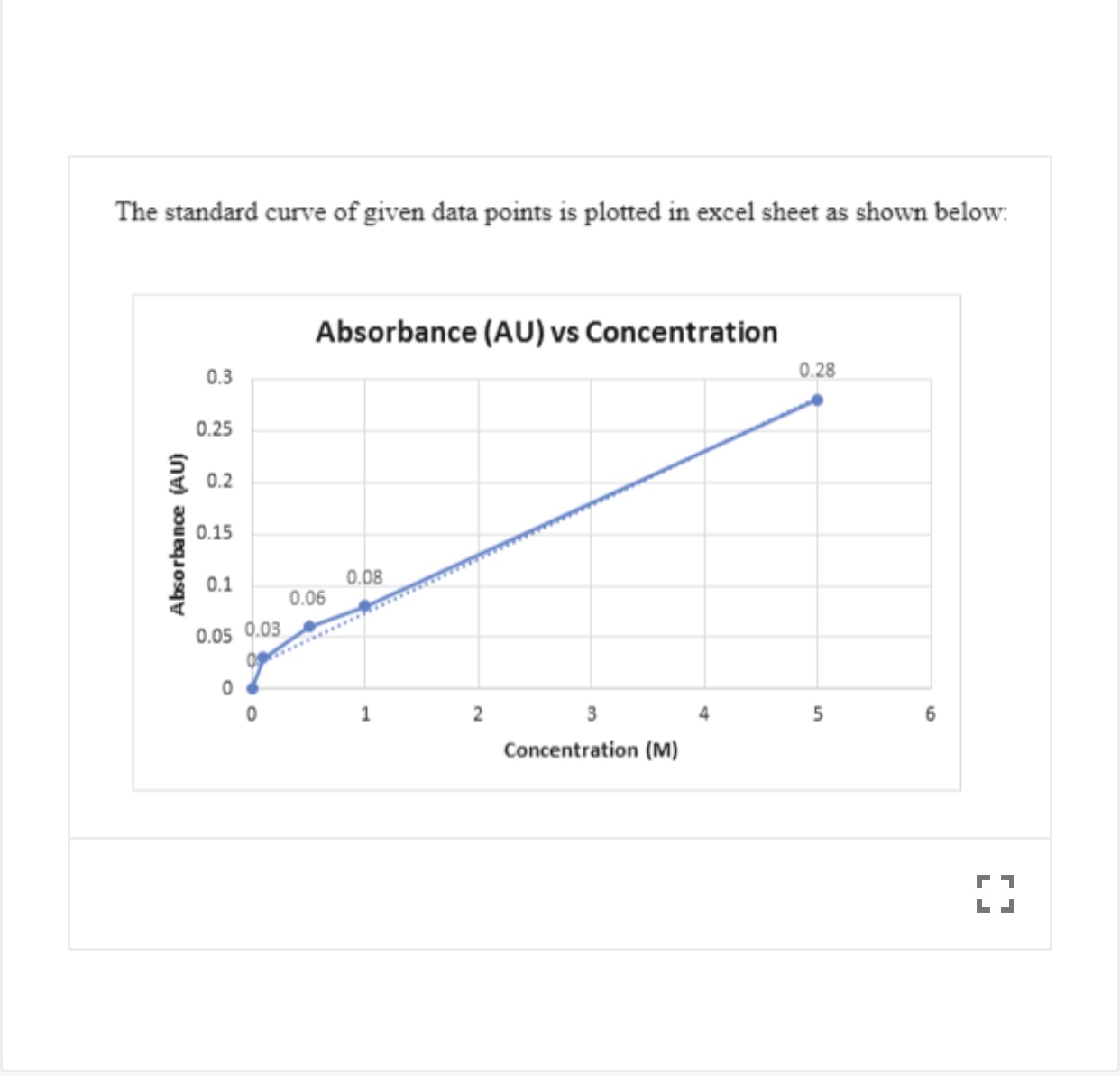
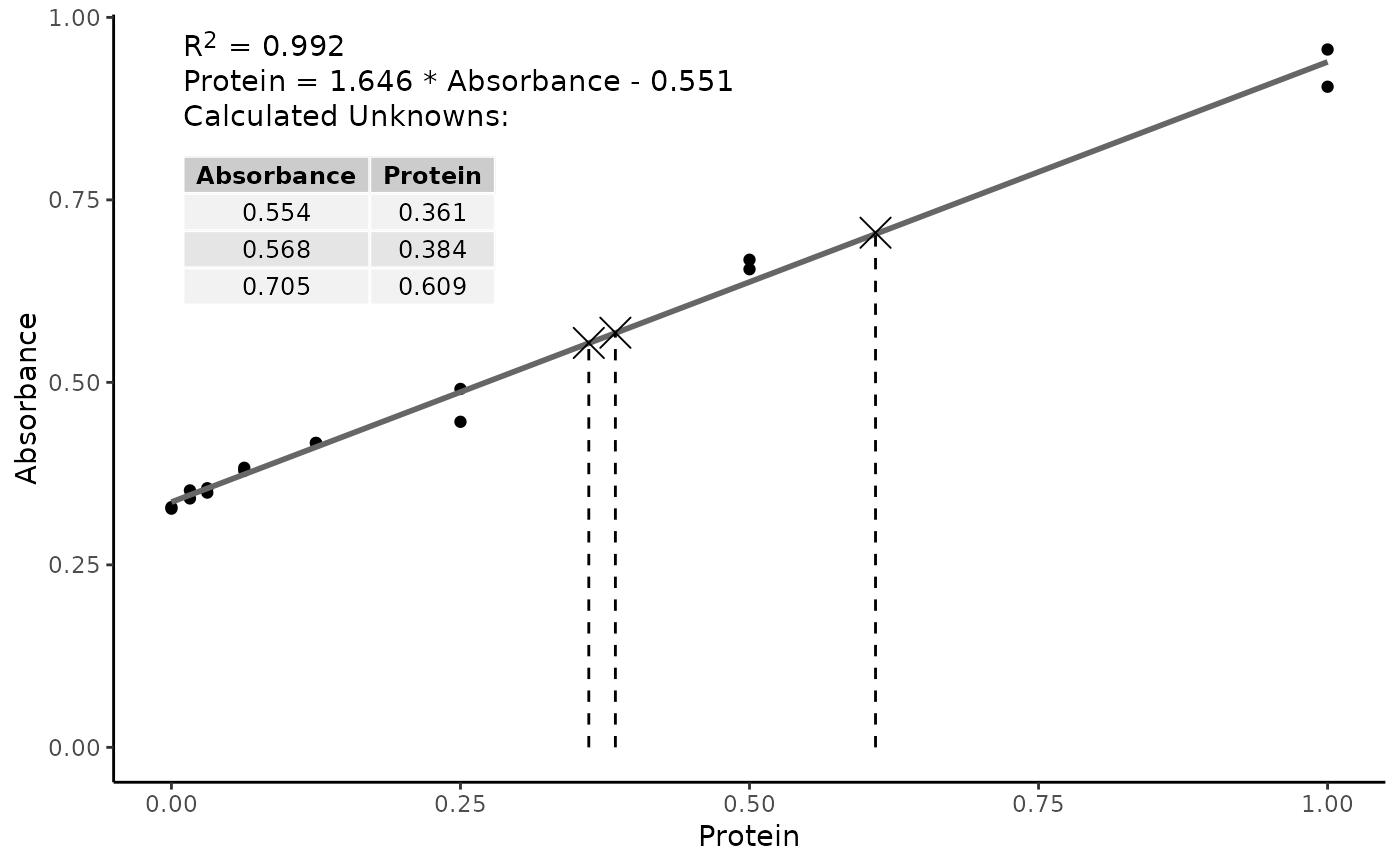
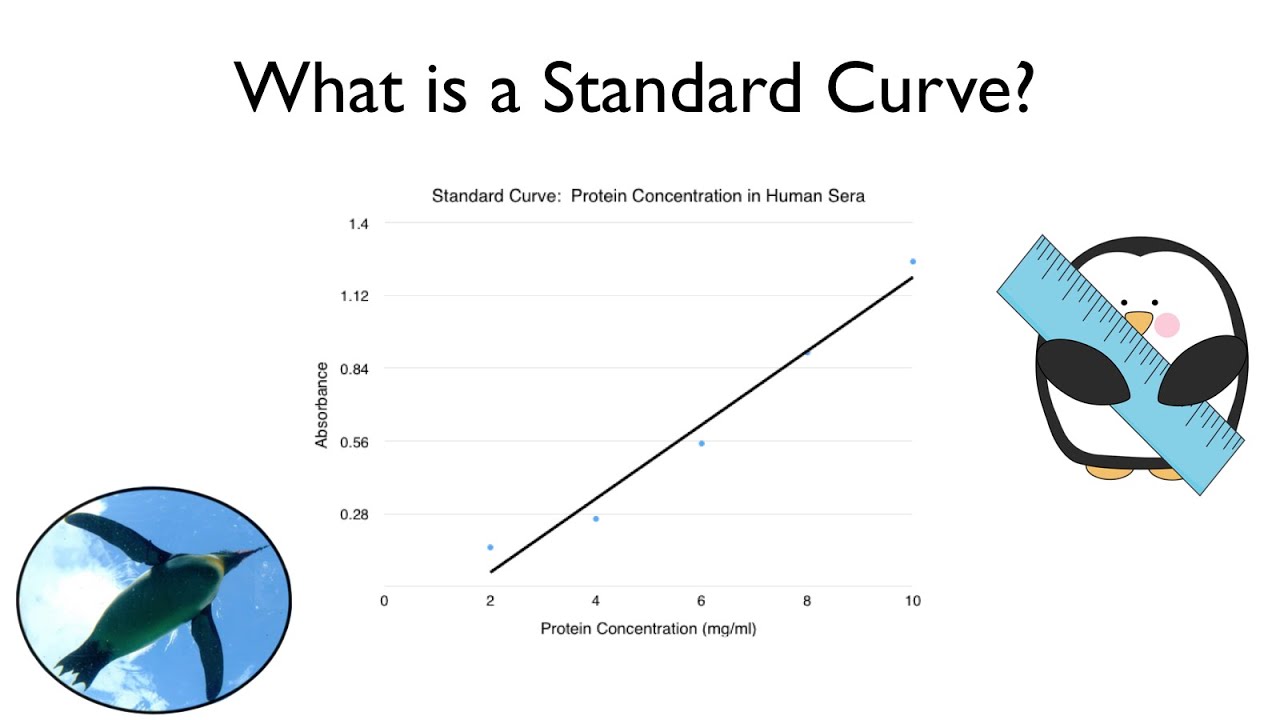
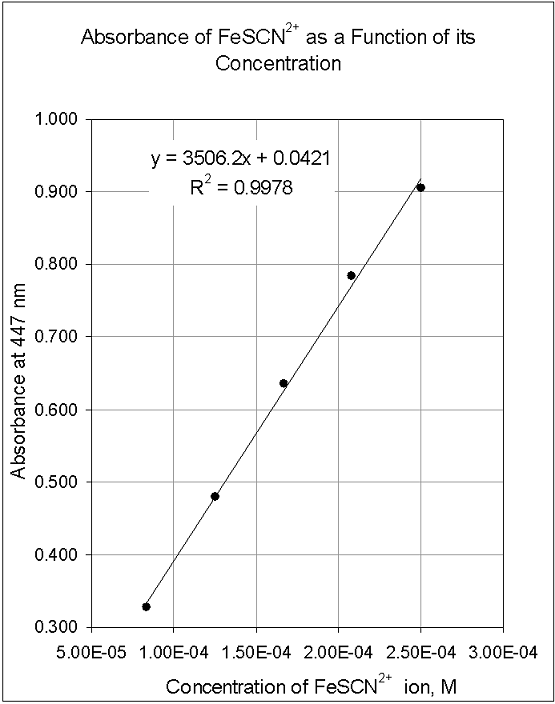
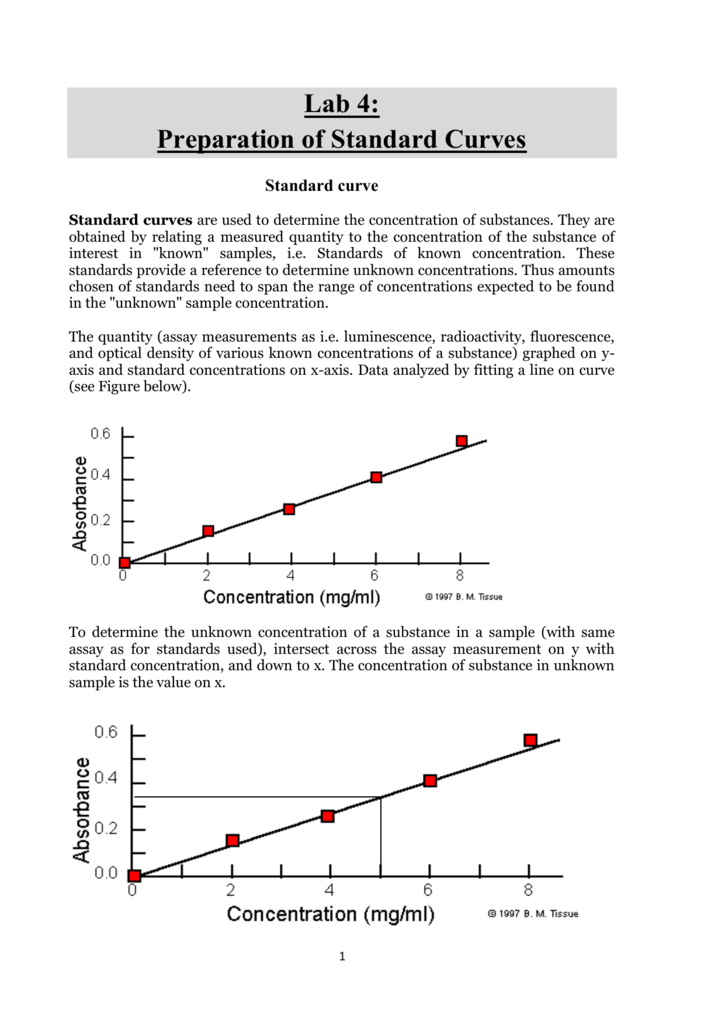
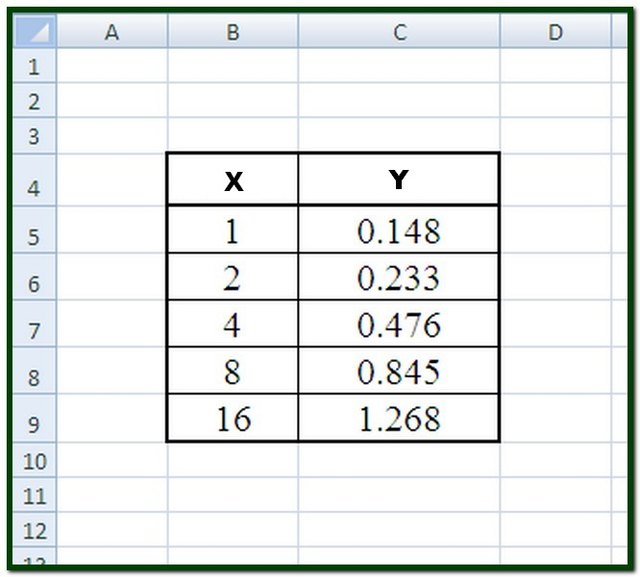
Preparation of the standard curve. That means you will get a line graph similar to that shown below (it may flatten out at. The plot of the standards should be linear, and can be fit with the equation y=mx+b.
Standard curves are used in many assays in biotechnology. The two images below show the variations seen with. If you are making a calibration curve for $\ce{cuso4}$ where you plot absorbance against concentration, does the wavelength have to be exactly at the.
Simply put, a standard curve is a plot of known x and y values. The calibration curve is a plot of instrumental signal vs. These curves use data points of known.