Painstaking Lessons Of Tips About What Is The Standard Curve Line Graph How To Add Equation Scatter Plot In Excel
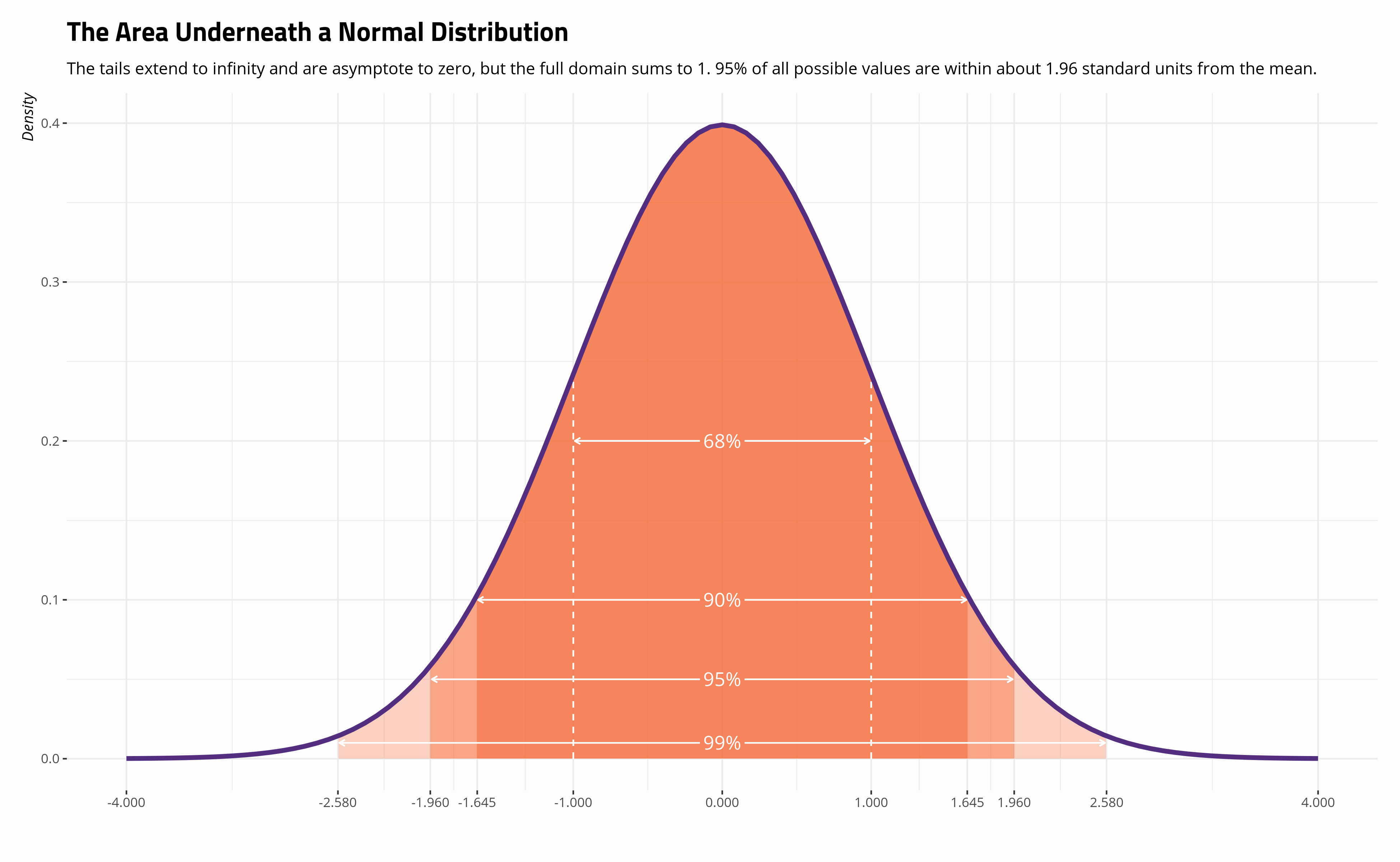
A graph in which one axis is a linear scale while the other axis is a.
What is the standard curve line graph. Standard curves are needed for many analyses in the lab including (but not limited to): Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. Linear regression of straight line calibration curves.
How can you determine the amount of drug released from a pill, the amount of pollutant. Explore math with our beautiful, free online graphing calculator. Be certain to include all elements of a good graph (title, axes labels,.
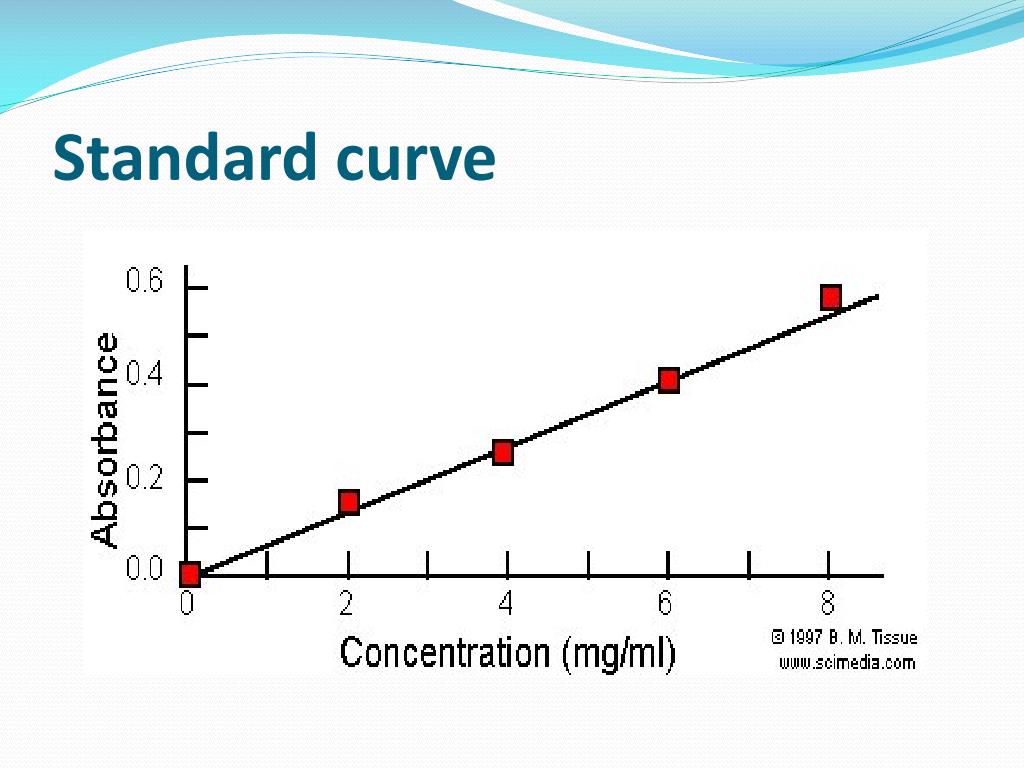
You should have a data set which was used to create a standard curve. Standard curves (also known as calibration curves) represent the relationship between two quantities. In analytical chemistry, a calibration curve,.
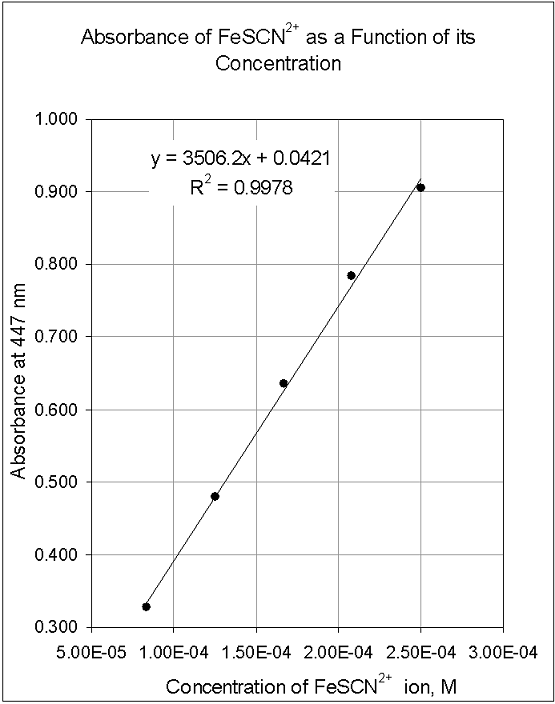
Release profiles, encapsulation efficiency calculations, greiss assays, and elisas. Example standard curve involving six points. However, not all curves are linear and sometimes to.
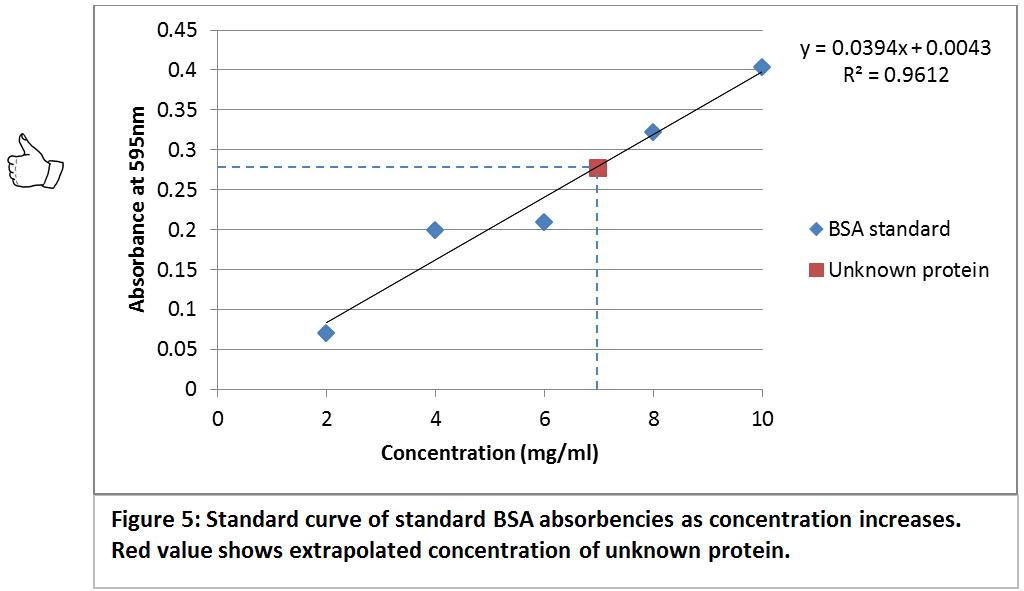
Use a standard curve to determine the values for unknown solutions. Standard curves are usually used in the field of biology to help you identify how much stuff (in many cases, this is the concentration of. Calculating unknown concentrations using a standard curve in prism 3.
A calibration curve plot showing limit of detection (lod), limit of quantification (loq), dynamic range, and limit of linearity (lol). A standard curve is a graph relating a measured quantity (radioactivity,. The thick line is linear regression for the entire set of.
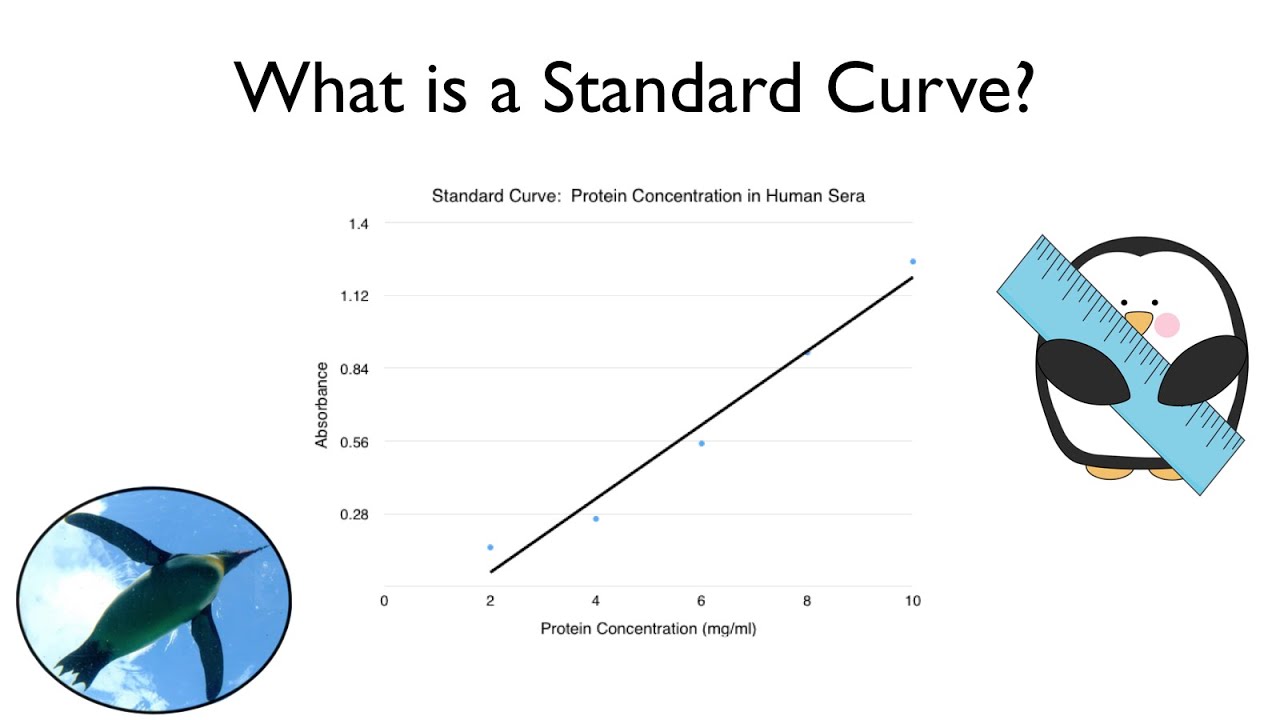
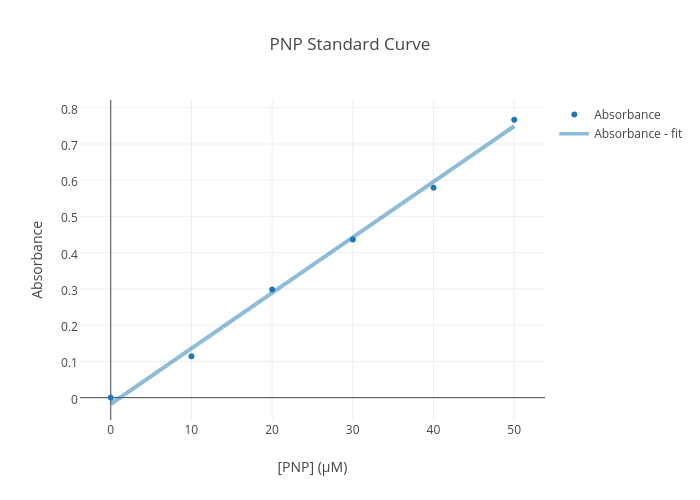
You need to produce a graph showing the linear relationship between the od of the solutions (e.g. The traditional method for calculating protein concentration of an unknown sample is to use a standard curve that is generated from known protein standards. Create a standard curve by graphing the following data (absorbance vs.
A standard curve is a graph relating a measured quantity (radioactivity, fluorescence, or optical density, for example) to concentration of the substance of interest in. The signal is never perfectly proportional to the.